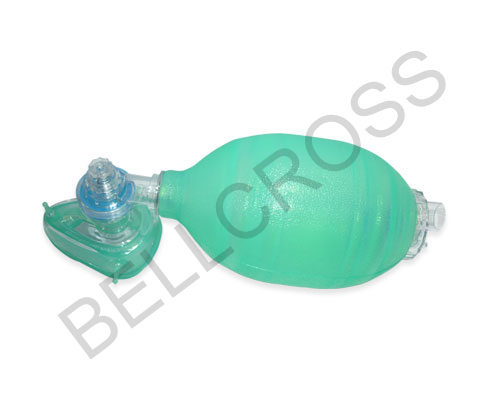
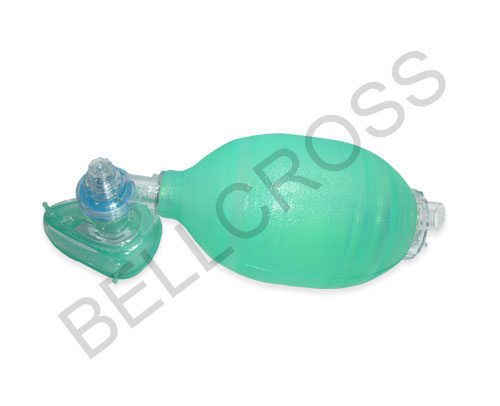
About Silicone Resuscitator Infant
Procure the Silicone Resuscitator Infant, a majestic leap in neonatal care, manufactured for formidable performance in emergency situations. With a 600 ml oxygen reservoir, pressure-limiting pop-off valve at 40 cm H2O, and laureate grip design, this non-toxic silicone device ensures precise tidal volumes. The translucent blue oval bag delivers sizzling accuracy while meeting ISO and CE standards. Autoclavable at 121C, latex-free, and reusable, this resuscitator suits hospital applications and guarantees best price with premium quality from Indias leading suppliers.
Application Surface and Direction of Use
The Silicone Resuscitator Infant is designed for manual resuscitation of infants and neonates, with a textured surface for a secure grip. To use, position the mask over the face, ensuring the oval bag covers both the mouth and nose. Adult supervision is essential during application, and the resuscitator is suitable for hospital site usage. Direction for use follows manual bag compression to deliver controlled tidal volume to the respiratory tract of neonates.
Packaging Details and Market Value
Premium shipped goods are delivered in protective medical packaging to maintain integrity. The Silicone Resuscitator Infant holds significant market value in Indias health sector, suited for hospitals and clinics. Main domestic market is India, with samples available for evaluation before procurement. Suppliers ensure every shipment meets compliance and quality standards to retain the laureate reputation in neonatal respiratory solutions.
FAQs of Silicone Resuscitator Infant:
Q: How is the Silicone Resuscitator Infant operated during emergency ventilation?
A: The device is manually operated by compressing the silicone oval bag, which delivers precise tidal volume through the mask to the infants airway, under adult supervision.
Q: What safety features are integrated into the resuscitator?
A: It has a pressure-limiting pop-off valve at 40 cm H2O to prevent over-inflation, a latex-free build, and is fully autoclavable for safe reuse.
Q: When should the Silicone Resuscitator Infant be sterilized?
A: Sterilize the device after each use by autoclaving at 121C, ensuring its safe and ready for subsequent applications.
Q: Where is the main application site for this resuscitator?
A: The primary application site is hospitals and neonatal units, where the device is used for manual respiratory support of infants and neonates.
Q: What benefit does the textured grip provide during procedures?
A: The textured surface ensures a firm grip for clinicians, reducing the risk of accidental slips and allowing optimal control during manual ventilation.
Q: How does the product meet international compliance standards?
A: The Silicone Resuscitator meets ISO and CE certifications, confirming its safety, quality, and efficacy for neonatal respiratory resuscitation.



 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese
 Send Inquiry
Send Inquiry