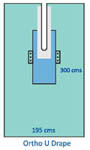
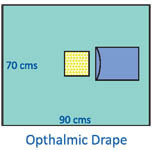
About Ophthalmic Drape
Discover our best-seller Ophthalmic Drape, renowned for its unprecedented protection and patient comfort in surgical settings. Engineered with SMS non-woven fabric and a precisely cut 7 x 9 cm adhesive aperture, this single-use drape is fluid and tear resistant, boasts high absorbency, and is both hypoallergenic and latex-free. Its ultrasonically sealed edges, medical-grade adhesive, and waterproof design ensure a glorious level of reliability for ophthalmic procedures. Housed in an individually packed sterile pouch, its ephemeral yet effective performance makes it a terrific asset for any hospital.
Unmatched Features and Utilization in Ophthalmic Procedures
This ophthalmic drape stands out through its ultrasonically sealed edges, fluid collection pouch, and medical-grade adhesive aperture. Specifically designed for ophthalmic surgeries, it is favored by experienced surgeons and hospital staff in operation theatres nationwide. The sterile, lightweight, non-irritant material guarantees comfort for patients while maintaining the integrity of the surgical environment. Its high absorbency, radiolucence, and disposable design make it ideal for delicate eye procedures, offering superior utility where precision and safety are paramount.
Certifications, Domestic Reach, and Seamless Handover
Certified with CE marking and ISO 13485, our ophthalmic drape assures global compliance and hospital-grade assurance. The primary domestic market is India, with major FOB port operations facilitating prompt delivery and systematic handover to clients. The investment outlay per individually packed piece translates into reliable, sterile, and ready-to-use protection. Our process ensures each drape is sterilized using ETO and shipped under stringent quality parameters, delivering peace of mind to healthcare providers nationwide.
FAQs of Ophthalmic Drape:
Q: How does the ophthalmic drape provide enhanced safety during surgeries?
A: The drape is made from SMS non-woven fabric, features tear and fluid resistance, and is latex-free and hypoallergenic, ensuring a sterile, protective barrier with ultrasonically sealed edges and high absorbency. These qualities minimize infection risks and keep the surgical field dry.
Q: What is the process for using the drape in ophthalmic operations?
A: Simply open the sterile packaging, peel off the backing from the medical-grade adhesive aperture, and position the drape so the aperture aligns with the surgical site. The fluid collection pouch and sealed edges keep the area dry and contained throughout the procedure.
Q: When should this ophthalmic drape be used?
A: This drape is specifically intended for ophthalmic surgeries and eye procedures performed in operation theatres. Its features make it suitable whenever a sterile, fluid-resistant, and hypoallergenic surgical environment is required.
Q: Where can these drapes be sourced?
A: These CE-marked, ISO 13485 certified ophthalmic drapes are manufactured and supplied across India, with distribution coordinated through major FOB ports, ensuring timely handover and delivery to hospitals and healthcare facilities.
Q: What are the benefits of choosing this particular drape over standard options?
A: This drape offers a combination of unprecedented fluid and tear resistance, patient comfort, hypoallergenic properties, and a medical-grade adhesive aperture, providing dependable and safe coverage for sophisticated eye surgeries.



 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese
 Send Inquiry
Send Inquiry